Key take aways from ASCO22
Topic: News

By: Sejin Chung
Hi! I’m Sejin Chung, PhD, the newest member of the Lustgarten Research Team. I was thrilled to represent the Foundation at ASCO22 to share a few key learning from various talks and poster sessions on pancreatic cancer. ASCO is important because exciting studies have data fresh off their trials and the researchers lead enlightening discussions about future directions for pancreatic cancer. I met several Lustgarten-funded investigators such as Dr. Vinod Balachandran from the David M. Rubenstein Center for Pancreatic Cancer Research.

Dr. Balachandran and his team’s work stirred up a lot of excitement in the pancreatic cancer field because it demonstrated how the immune system can protect the patient from recurrence. It was also promising for the clinic as the vaccine development and administration were completed in a clinically relevant timeframe. His study in the mRNA vaccine revolved around the hypothesis that individualized mRNA neoantigen vaccines can induce neoantigen-specific T cells and delay recurrence in PDAC patients. The vaccine, autogene cevumeran, was shown to be safe and highly immunogenic in 50% of their patients. To note, the vaccine was able to durably expand T cells that correlated with delayed recurrence. Their study was supported by Lustgarten, and it was exciting to hear about the success of their study as well as follow up studies that will occur.
Dr. Kim Reiss (University of Pennsylvania) had a poster illustrating their randomized phase Ib/II study of a PARP inhibitor (Niraparib), and a PD-1 inhibitor (Nivolumab), or a CTLA-4 inhibitor (ipilimumab), in patients with platinum sensitive advanced pancreatic cancer. She is from Dr. Robert Vonderheide’s research group at University of Pennsylvania, who is currently co-funded by Lustgarten and Stand Up To Cancer.
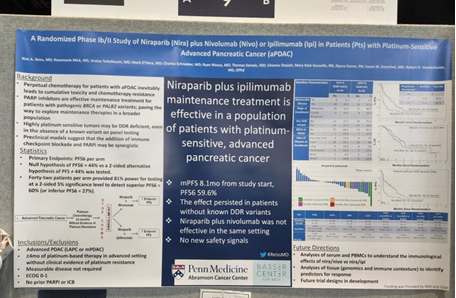
The treatment regimen involved platinum chemotherapy for more than 4 months without evidence of resistance in advanced pancreatic cancer patients. The chemotherapy treatment was stopped prior to the combination arms of Nira + Nivo or Nira + Ipi. Results showed that niraparib and ipilimuab treatment was effective in patients sensitive to platinum. Successful synergy was seen in patients without known DNA damage repair variants, which could be useful for future stratification. In a later discussion session led by Dr. Arsen Osipov (Cedars-Sinai Medical Center), he notes the key takeaway is that immunotherapy combinations with unique adjunctive agents are still reasonable pathways for clinical investigation. Besides targeting KRAS, he suggests looking at the tumor microenvironment and chemoresistance.

Dr. Neeha Zaidi who works with Dr. Liz Jaffee, Lustgarten’s Chief Medical Advisor, gave an excellent talk on using the immune system against mutant KRAS in pancreatic cancer. Mutant KRAS drives immune evasion and mutated KRAS antigens are ideal targets because they are derived from common hotspot mutations. Dr. Zaidi mentioned current vaccine trials, including the Lustgarten-funded KRAS peptide vaccine study. Another strategy is to utilize genetically modified T cells to target mutant KRAS, recently published in the New England Journal of Medicine. To close her talk, Dr. Zaidi highlighted combination strategies including checkpoint inhibition and KRAS inhibitors that can be used in advanced tumors and immunologically “cold” tumors such as PDAC.
Dr. Andrew Aguirre, a Lustgarten-funded scientist, at Dana Farber Cancer Center gave the final talk in this session. He presented on small molecule inhibition of KRAS. For pancreatic cancer, there are multiple RAS inhibitors currently in development, including inhibitors for the G12D mutation. Although the G12C mutation is not prevalent in PDAC, Dr. Aguirre presented a proof-of-concept study that showed KRAS inhibition of G12C mutation is effective. However, monotherapy is not going to be the answer, and combination therapies with immunotherapy, chemotherapy, and synthetic lethal targets will be promising.

I also attended the GI Cancer session, “Can We Begin to Predict Responders to Targeted Therapy in Gastrointestinal Cancer?” During this session, Dr. Lacey Padron (Parker Institute for Cancer Immunotherapy) presented results from the PRINCE trial which was a study testing drug combinations in metastatic PDAC. The drug combinations were: CD40 agonistic monoclonal antibody (APX005M/sotigalimab) + anti-PD-1 antibody (Nivolumab) + Chemotherapy (Gemcitabine + nab- Paclitaxel), Sotigalimab + chemotherapy, and Nivolumab + chemotherapy. The primary endpoint of 1-year overall survival (OS) rate was 57.7% for nivo/chemo, 48.1% for sotiga/chemo, and 41.3% for the triple combination arm. The most important analysis of this study is that patients with longer survival after the double combination regimens could be identified by predictive biomarkers from the blood and the tumor. Their finding encourages more effort toward characterizing patients that would benefit from the combination.
On the topic of predicting responders, Dr. Jennifer Knox (Princess Margaret Cancer Centre) another Lustgarten funded scientist, and her team, presented “Molecular characterization of long-term and short-term survivors of advanced pancreatic ductal adenocarcinoma” in the poster session.
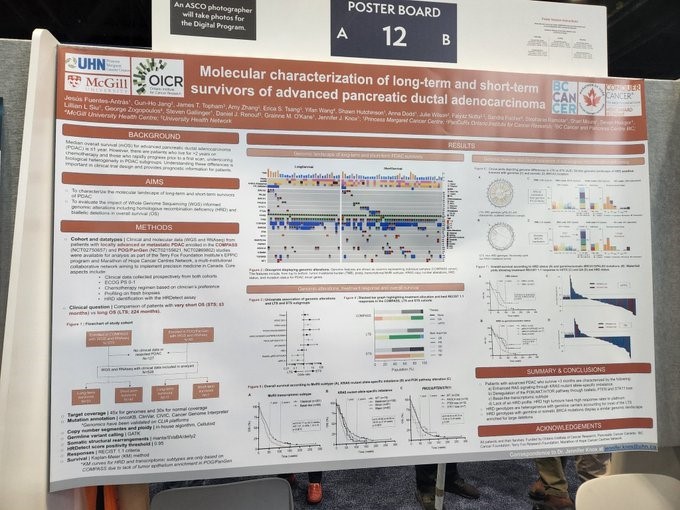
They sought to characterize the molecular landscape of survivors with locally advanced or metastatic PDAC and to evaluate the impact of whole genome sequencing informed genomic alterations in overall survival. In summary, they found that patients with advanced PDAC who survive less than 3 months lacked a homologous recombination deficiency (HRD) profile, whereas the long-term survivors had HRD genotypes that were heterogeneous with germline carriers.

Another great session was the joint ASCO and AACR session, “The Promise of DNA Damage Response and Repair in Cancer” that was co-chaired by Dr. David Tuveson, Lustgarten’s Chief Scientist. Dr. Alan Ashworth kicked off the presentations by discussing the history of harnessing DNA damage repair deficiency in cancer treatment. He presents the synthetic lethality strategy, with PARP inhibitors in BRCA1/2 mutated cells as an example. Currently, there are various PARP inhibitors approved by the FDA for multiple indications, including pancreatic cancer. But, it is not a surprise that resistance to PARP inhibition can occur, and combination with other agents such as immunotherapy agents may enhance activity (i.e. the study by Dr. Reiss).
The last speaker was Dr. Lillian Siu, who talked about clinical trials and targets beyond BRCA. Although BRCA is a gene associated with homologous recombination, other genes are associated as well: ATM, ATR, PALB2, and CDK12. Regarding clinical trials, there are many inhibitors that are in the clinic to test these non-BRCA genes as targets. In summary, there are many trials testing the efficacy of targeting DNA damage repair deficiency, but patient selection biomarker strategies are variable. Unsurprisingly, more research is needed!
ASCO 2022 was a wonderful conference full of great research and discussion. I was grateful to attend and happy to have seen some ground-breaking research that was funded by Lustgarten!


