AACR Summary
Topic: News

Earlier this month, expert pancreatic cancer researchers and young investigators gathered in Boston for the AACR Special Conference on Pancreatic Cancer. With over 600 registered in-person attendees and over 230 abstracts submitted for presentation, it was an event full of ground-breaking research and researchers from all levels. The Conference was chaired by Andrew Lowy, MD, FACS from the UC San Diego; Marina Pasca Di Magliano, PhD, from the University of Michigan Medical School; Robert Vonderheide, MD, from Penn Medicine; and Jen Jen Yeh, MD, from the UNC Lineberger Comprehensive Cancer Center University of North Carolina, Chapel Hill. It was truly a remarkable experience filled with great pancreatic cancer research and excitement.

The meeting kicked off with a welcome from Dr. Lowy and Andrew Rakeman, PhD, Vice President of Research at Lustgarten Foundation. As a representative for Lustgarten, Dr. Rakeman spoke of the remarkable progress that has been made and the importance of continued research to transform pancreatic cancer into a curable disease.
Avery Posey, Jr, PhD, from the University of Pennsylvania, the 2021 recipient of the Lustgarten Foundation John Lewis -Career Development Award, gave the Rising Star Lecture. Dr. Posey’s lecture reviewed variable factors in generating chimeric antigen receptor (CAR) T cell therapy in pancreatic cancer, one of which is truncated O-glycans. Dafna Bar-Sagi, PhD from NYU Langone Medical Center gave the Opening Keynote. Dr. Bar-Sagi reviewed the contextual determinants of pancreatic tumorigenesis and highlighting work supported by the Lustgarten Foundation and Stand Up to Cancer grant.
The first plenary session focused on disparities and health equity. Karen Winkfield, MD, PhD, from Meharry-Vanderbilt Alliance discussed the disparities in incidence and outcomes in pancreatic cancer, highlighting the populations at greatest risk of exclusion from care and clinical trials. She reminded the audience that it is important to consider environmental and epigenetics as a source of disparity rather than focusing only on genetics. Dr. Winkfield stated that this is an opportunity to consider addressing access and inclusion at all steps, from research to clinical trial and execution to care. The selected abstract speakers during this session provided evidence of race-specific differences in pancreatic tumors and drug response, further strengthening the need for more inclusion in pancreatic research.
The session concluded with Cleo Samuel-Ryals, PhD, from Flatiron Health with her talk, “System Level Approaches to Cancer Care Equity.” In addressing inequities in clinical trial participation, Dr. Samuel-Ryals suggested promoting widespread information about clinical trials to all eligible patients, policy and regulatory changes, and leveraging data to inform trial design. This session was a great reminder of why grants such as Lustgarten Equity, Accessibility, and Diversity (LEAD) are critical to address barriers to clinical trial participation in minority patient populations.
Next sessions included talks on preclinical studies that led to clinical trials, updates on recent clinical trials, tumor plasticity, and drug resistance. Katelyn Byrne, PhD, from Oregon Health and Science University reviewed preclinical data conducted during her postdoctoral training with Dr. Vonderheide, where they studied how the agonist CD40 alters the tumor microenvironment and immune response. They found that in a mouse model of pancreatic cancer, CD40 agonist with chemotherapy and immune checkpoint inhibitor was effective in reducing tumor size. Dr. Vonderheide discussed the clinical data derived from this preclinical work, focusing on the PRINCE trial and lessons learned. Their phase 1 trial, supported by Lustgarten, showed promising results and led to the phase 2 expansion that had three different treatment arms (combination of chemotherapy, immune therapy, and CD40). The PRINCE study failed to show a benefit from CD40 but found immune markers that predict response to CD40 or immune checkpoint inhibitors. His talk ended with a call to action for precision oncology approaches.

Alicia Braxton, DVM, DACLAM, from understanding gave an exciting talk about pancreatic intraepithelial neoplasia (PanIN). Using a novel 3D modeling technique called CODA, they were able to analyze human PanINs from surgical samples. CODA automatically segments the cell types in the tissue and can distinguish normal duct from PanIN. Her work revealed that PanINs are multifocal and arise from independent events, which was unexpected. Future work will be to understand what drives some of the PanINs to progress to pancreatic cancer. As early events are critical to understanding and diagnosing pancreatic cancer, CODA is a novel technology that provides the opportunity to dissect surgical tissues. This technology will be utilized by Laura Wood, MD, PhD, at Johns Hopkins Medical Institute, who is a grant recipient from Lustgarten, to improve the early detection of pancreatic cancer.
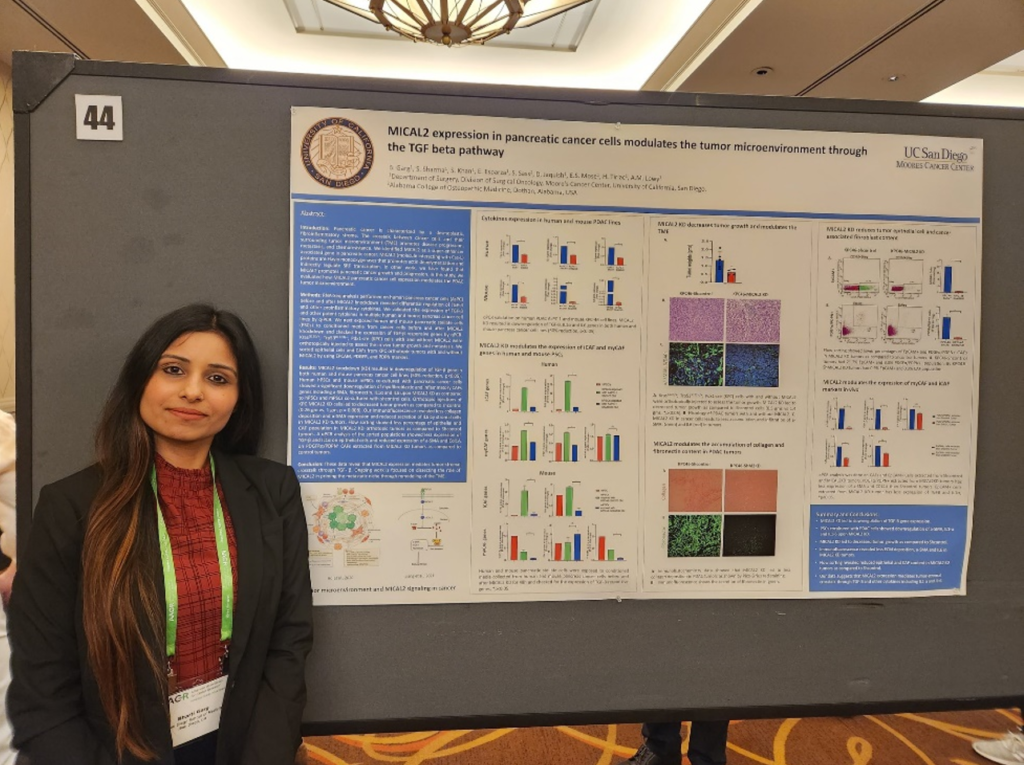
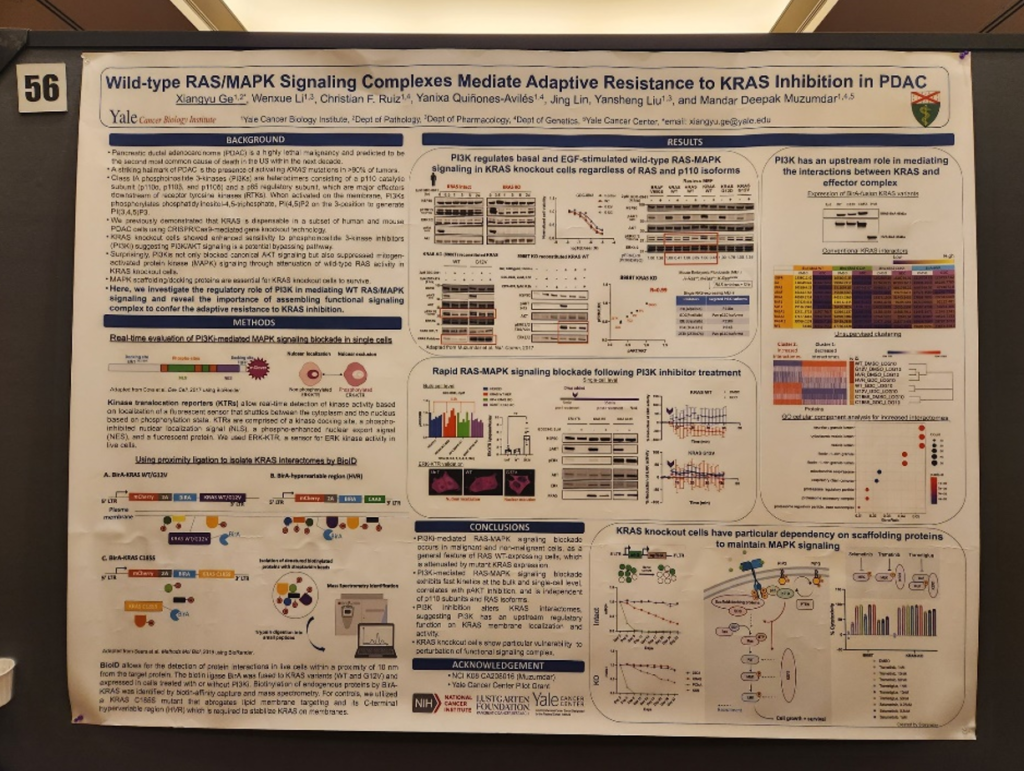
We also had a chance to listen to poster session presentations that were held at the end of each day. It was great to see so many young investigators highlighting their work beside their mentors I spoke with Bharti Garg, PhD, who works with Dr. Lowy at the UC San Diego. Their Lustgarten funded project focused on MICAL2 expression and how it modulates the tumor microenvironment in pancreatic cancer. Knock out of MICAL2 in a pancreatic cancer mouse model led to decreased control compared to control, and their data suggested MICAL2 expression mediates tumor-stromal crosstalk. In addition, Lustgarten grantee Mandar Muzumdar, MD, from Yale University had multiple posters from his trainees. One poster highlighted their Lustgarten-funded work regarding the role of wild-type RAS/MAPK signaling in KRAS inhibition in pancreatic cancer. This work is important as we know KRAS is difficult to target, and new KRAS inhibitors mean more resistance mechanisms that could follow. Lastly, Dannielle Engle, PhD’s lab from the Salk Institute presented work in glycan CA19-9 mediated remodeling of the pancreatic tumor microenvironment. Their work was supported by Lustgarten and they found CA19-9 elevation induces cancer associated fibroblast (CAF) differentiation. These posters represent only a few of the multitude of great works that the young investigators are working on for pancreatic cancer.
Plenary session 4 began with Theodore Hong, MD, of Mass General Research Institute and the Lustgarten Scientific Advisory Board, who reviewed clinical data on neoadjuvant therapy to increase the rate and success of surgery for pancreatic cancer patients. Only 15% of patients present with resectable disease and only 3% of those patients will be cured by surgery. Thus, better approaches are needed, and efforts should be focused on increasing resectability, not ablation. Additionally, he commented that we need a consensus on surgical decision-making to improve patient outcomes. Philip Philip, MD, from Henry Ford Cancer Center also discussed the challenges of locally advanced pancreatic cancer. He encouraged researchers and clinicians to look for solutions in moving more quickly for combinations and personalized approaches. Specifically, he stated that we need biomarkers to match patients and therapy and approaches for rationale combinations to be able to do clinical trials in smaller number of patients.
Dr. Yeh, also a member of the Lustgarten Scientific Advisory Board, closed the session with her talk on precision oncology approaches in pancreatic cancer. She outlined the current strategies of diagnosis and non-invasive biomarkers including CA19-9 and imaging. She noted that we need better biomarkers to tailor current therapies based on patient characteristics. Dr. Yeh closed her talk by calling for opportunities to improve understanding of diagnosis, selection of treatment pre/post-surgery, and understanding of how and when to treat.
In the Prevention and Interception session, there were talks from young investigators who highlighted ongoing studies to prevent the progression of pancreatic cancer. Christian Ruiz, PhD, from Yale University and Dr. Muzumdar’s lab presented the role of excess dietary oleic acid in pancreatic cancer progression. They attempted to understand the link between obesity and pancreatic cancer risk by investigating fat intake in mouse models. They found that fats with high oleic acid had highest risk of tumor burden and dietary oleic acid directly induced acinar-to-ductal metaplasia (ADM). Their work was funded by Lustgarten.
Additionally, Neeha Zaidi, MD, from Sidney Kimmel Comprehensive Cancer Center presented work from Elizabeth Jaffee, MD, Lustgarten Chief Medical Advisor, on intercepting pancreatic cancer development with mutant KRAS-targeted immunotherapy. Dr. Zaidi reviewed the rationale for targeting prevention in pancreatic cancer by using a mutant-KRAS vaccine approach. The window of opportunity for interception is wide in pancreatic cancer because a decade passes from the first KRAS mutations until the appearance of pancreatic cancer. They have completed their vaccine clinical trial in resected patients and observed good immunogenicity. This led to their vaccine clinical trial in high-risk individuals, which currently has 3 patients vaccinated and immune response measured in the first two patients. Their work was supported by Lustgarten to improve pancreatic cancer prevention strategies.

Kimberly Perez, MD, from Dana-Farber Cancer Institute provided an update on the role of vitamin D receptor agonist in the treatment of pancreatic cancer in the Metabolism and Microbiome session. Funded by the Stand Up to Cancer and Lustgarten grant, their work investigated Vitamin D Receptor (VDR) as a target in pancreatic cancer. Preliminary data showed that a combination of VDR agonist and chemotherapy improved survival in mice and increased access of chemotherapy to the tumor. This led to their clinical trial (NCT03520790) which has shown to be well tolerated. The study has been completed, but the treatment arms are still blinded, and correlative studies are ongoing.
Joshua Rabinowitz, MD, PhD, from Princeton University closed the session with his talk on diet, microbiome, and pancreatic cancer therapy. He discussed ATP production in pancreatic cancer and tumor lymphocytes in relation to diet and therapy. His lab observed how diet could factor into tumor dedifferentiation and found that keto diet with combination of triple chemotherapy led to significant tumor suppression. This led to a current clinical trial that is evaluating the ketogenic diet in patients with metastatic pancreatic cancer (NCT04631445).

The conference ended with a debate between Sunil Hingorani, MD, PhD, from Fred and Pamela Buffett Cancer Center and Howard Crawford, PhD, from Henry Ford Pancreatic Cancer Center. Tasked with the topic of targeting the tumor vs. the stroma, Dr. Hingorani took the stroma position and discussed principles and predictions from the model of stromal-epithelial co-evolution in pancreatic cancer. Dr. Crawford defined the tumor as a combination of the tumor and the stroma but emphasized the importance of targeting the tumor cells because that is where the mutations that drive pancreatic cancer are found. In the end, unsurprisingly, we know that both are significant in considerations for pancreatic cancer therapy.

Our thanks to AACR for organizing an amazing conference. As the lead supporter of the conference, it was gratifying to witness the work that the Lustgarten Foundation has funded through the years. From expert investigators to new, young researchers, there was an absolute consensus on the fight for pancreatic cancer therapy and the importance of improving patient lives.


