Clinical Trial Phases
Clinical trials are used to determine the safety and efficacy of new treatments or therapies, typically in relation to existing protocols. Safety is an important feature of all clinical trials, and efficacy is an increasing focus, alongside safety, a trial moves through the various phases. In addition to the increased focus on efficacy, trial phases also add a larger number of people as the phases progress.
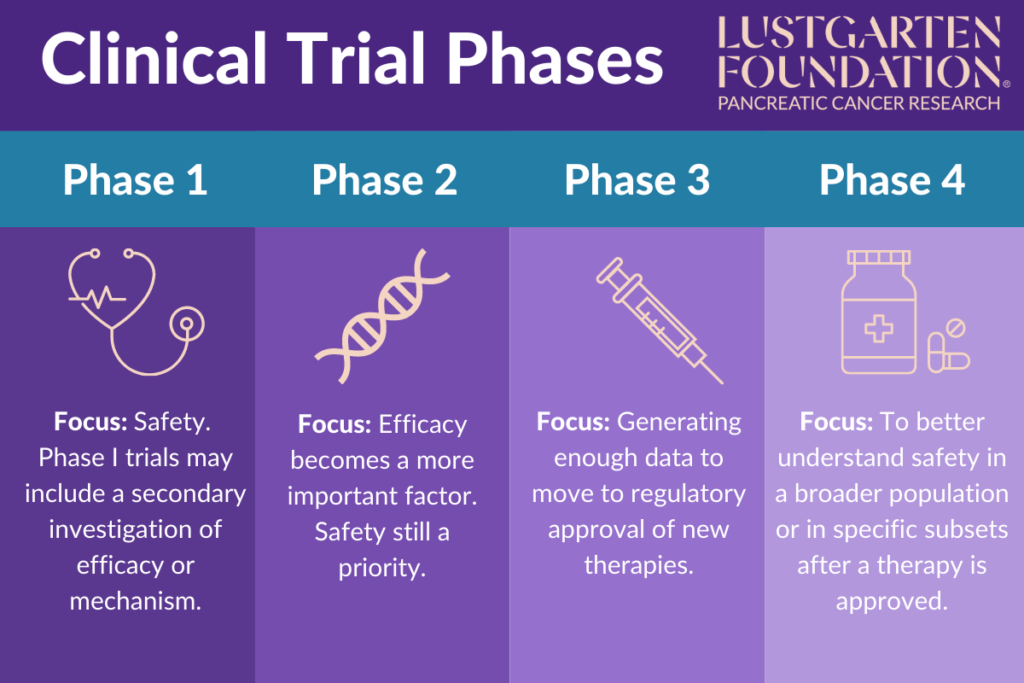
Phase I:
- Focus: Safety. Phase I trials may include a secondary investigation of efficacy or mechanism.
- Often tests multiple doses to determine the safest optimal dose.
- Can be the first time tested in humans or for a new indication or patient population.
- Outcome: If the therapy is shown to be safe, can move to Phase II
Phase II:
- Focus: Safety, but efficacy becomes a more important factor.
- Different doses are used to continue testing optimal dose/schedule
- Typically, a larger number of patients are treated than in Phase I.
- Outcome: If shown to be safe and have a benefit, can move to phase III, or additional phase II studies to continue to optimize the dosing or patient population
Phase III:
- Focus: To generate enough data to move to regulatory approval of new therapies.
- These are typically the largest studies; enrolling hundreds or thousands of patients depending on the drug, condition and design of the study.
- Continue to focus on safety.
- Increased focus on efficacy.
- Outcome: If successful, move to regulatory approval.
Phase IV:
- Focus: To look in the indication it is approved for to better understand safety in a broader population or in specific subsets after a therapy is approved.
- Looking at: how the therapy works, who it works best in and/or how it might interact with other conditions or other treatments, etc.
- Phase IV studies are sometimes also called “Post-marketing studies.”
- Outcome: The therapy can be studied for much longer period of time with a much larger population to see if any rare or unique side-effects occur.


