Interview with an expert: Radiation therapy update
Topic: Therapeutics
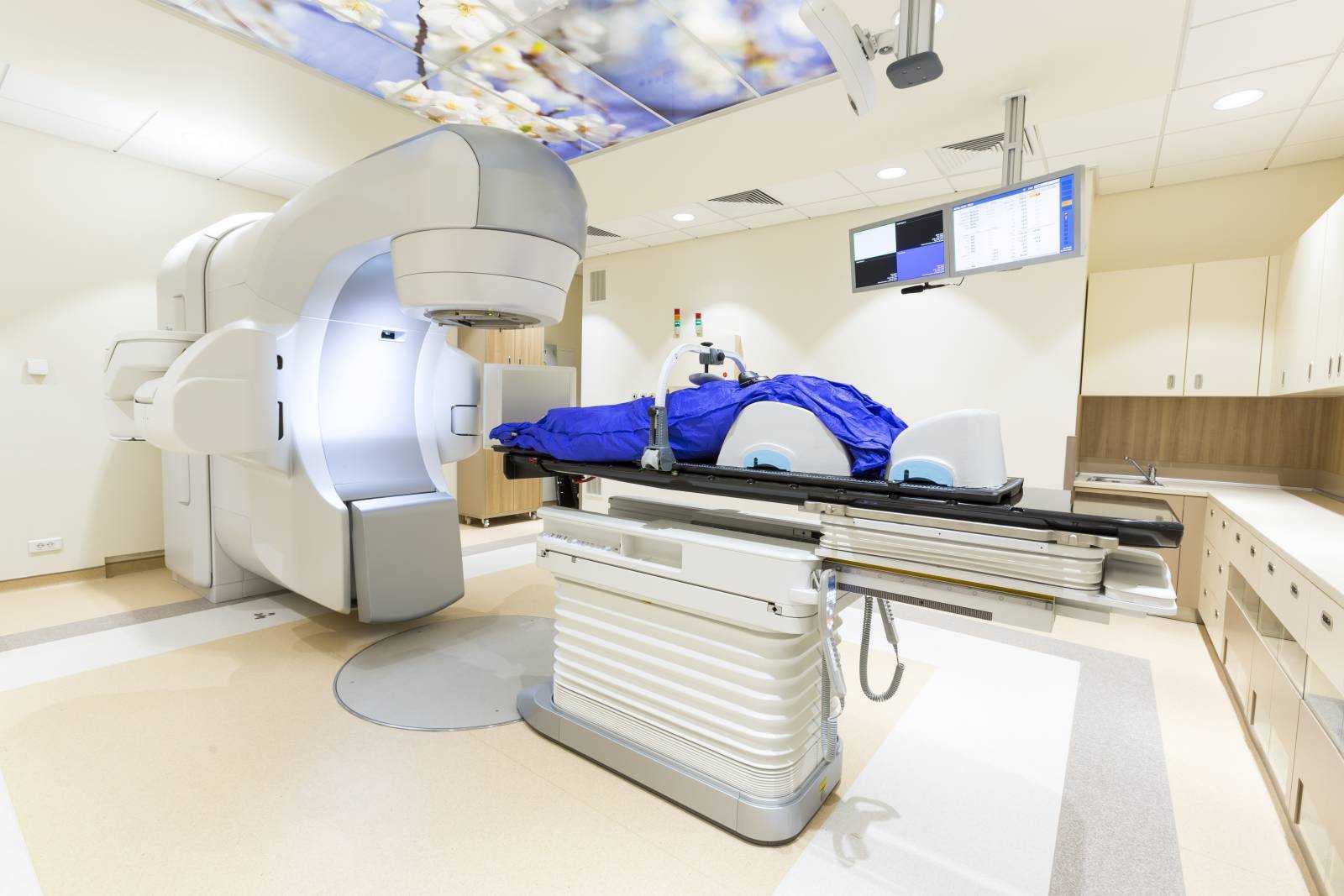
In the treatment of pancreatic cancer, radiation therapy (also known as radiotherapy), which involves a radiologist aiming a high dose of radiation toward a patient’s tumor to attack the cancerous cells, was once used only to relieve pain and other symptoms of the disease. However, due to a greater understanding of the biology of pancreatic cancer, increased knowledge of how radiation therapy alone or in combination with other treatments can affect patients, and the development of highly sophisticated technologies, radiation’s role in pancreatic cancer treatment is growing. Numerous clinical trials are currently ongoing that will help further define how radiation can help patients with pancreatic cancer at each stage of the disease.
How does radiation therapy work?
In radiation therapy, a radiologist aims a high dose of radiation toward a patient’s tumor. The radiation damages the fragile DNA of cells, which is the code of instructions that cells need to survive and do their job. Radiation can directly damage the DNA by causing breaks or tears along the genetic material, or it can trigger the body to form reactive molecules that will damage the DNA. The attack on the cells can be overwhelming, and ultimately those cells will die.
Doesn’t radiation have to travel through healthy tissue to get to a tumor?
Yes, radiation does have to travel through healthy tissue to reach a tumor, and there is a chance that non-cancerous cells become damaged by radiation. However, healthy cells have an entire arsenal to fix the DNA damage from radiation treatment. Conversely, cancerous cells, by their very nature, are faulty and can’t repair the DNA damage caused by radiation. One of the reasons radiation treatment is given across multiple sessions, generally spread out over a period of time, is to allow the healthy cells time to recover between treatments. It is crucial to minimize the risk and collateral damage to healthy cells, while maximizing the dose of radiation a tumor receives.
Radiation therapy has come a long way in the last couple of years, especially for pancreatic cancer patients. There used to be a lot of potential toxicity, but there have been such incredible improvements in technology, and some treatments are so focused and targeted that patients have few side effects. In addition, patients don’t see or feel radiation. It has a cumulative effect on the tumor, while hopefully sparing the healthy tissue.
How is radiation delivered to a patient?
One standard way we deliver radiation to pancreatic cancer patients is called chemoradiation, which combines a chemotherapy drug with radiation. It’s typically given over five-six weeks, Monday through Friday, and it takes about 15–30 minutes per treatment. It’s very effective in controlling and/or improving a surgeon’s ability to completely remove the pancreatic tumor.
Most radiation is external beam, meaning that a machine outside the body delivers the radiation. There are different forms of external beam radiation. Three-dimensional conformal radiation therapy, one of the most common types, uses computer software and sophisticated technology to deliver radiation doses to target areas very precisely. Another form is intensity-modulated radiation therapy (IMRT). With IMRT, there are hundreds of collimators, or radiation-shaping devices that are used to deliver a single dose of radiation.
The collimators can stay in place or move during the treatment, which enables us to control and change the intensity of the radiation beams during a treatment session, allowing us to better shape the radiation to the tumor while decreasing radiation to adjacent normal tissues.
Another standard way that we deliver radiation is stereotactic body radiation therapy (SBRT). With SBRT we can deliver multiple thin beams of high dose radiation therapy or focused “arcs” of radiation in fewer sessions. SBRT has evolved so that three to five days of radiation may be as effective as or even more effective than a five-and-a-half-week chemoradiation regimen. (More information on SBRT will follow later in this piece.)
Intraoperative radiation therapy is external beam radiation therapy that is delivered at the time of surgery. It is often delivered with electrons using a cone that focuses the radiation to the tumor while blocking normal tissues. This is often given as a boost to deliver an even higher dose of radiation therapy.
There are also other forms of radiation, but these are generally the most common. Used less frequently, internal radiation therapy or brachytherapy can be done by placing radioactive material inside the tumor. This can be done endoscopically (through a scope) or at the time of surgery.
Is radiation appropriate for every pancreatic cancer patient?
Every pancreatic cancer patient needs a multidisciplinary team, which includes a radiation oncologist. The team will collectively determine if and when radiation is appropriate. There are several different settings in which radiation oncology plays an important role, depending on the stage of the tumor; if surrounding structures, such as blood vessels, may be involved; and if a patient receives radiation before or after surgery.
The first setting in which radiation is appropriate is when a patient undergoes surgery because he or she is clearly a surgical candidate. We try to sterilize the margins of the tumor, or the area around the tumor, to decrease the chances of cancer returning. The second setting is when a patient has locally advanced disease (unresectable), when the cancer hasn’t spread to distant locations in the body, but it has moved into blood vessels around the pancreas, making it difficult for surgeons to completely remove the tumor. This is where we have made the most progress. Now, in certain situations, we can sometimes shrink the tumor enough so that patients can undergo surgery. Other times, the tumor doesn’t change size, but radiation kills the cells on the periphery of the tumor so that the surgeon can safely remove it from the vessels without leaving any viable cancer behind. Since surgery offers the best chance for long-term survival, having more patients qualify for surgery is an important goal.
Even when patients have significant local spread or metastases, radiation may be able to alleviate symptoms. Yet, despite our advancements in treating pancreatic cancer patients with radiation, we need more clinical trials and biomarkers to determine which patients are more likely to benefit.
Have there been trials supporting this approach for locally advanced disease?
Absolutely. Several trials found there was a significantly higher median survival for radiation plus a chemotherapy treatment called 5FU or 5-fluorouracil. Ongoing studies focus on optimizing how 5FU is delivered with radiation. There are also additional trials underway to determine how best to combine different radiation regimens with chemotherapy and targeted agents. Deciding which patients should receive chemoradiation depends largely upon the location and stage of the tumor as well as their disease characteristics. I believe that if a patient suffers from local obstruction or severe abdominal pain, then chemoradiation or SBRT is generally the best choice to alleviate symptoms. There are trials suggesting that this approach may increase the number of patients who can undergo surgery.
What about radiation for those patients who are surgical candidates, and those who are considered “borderline” surgical candidates?
A tumor that is resectable is usually away from adjacent blood vessels, or the surgeon can remove the tumor and the blood vessels safely. Potentially resectable or borderline resectable patients have tumors that involve blood vessels. However, they could possibly be surgical candidates but require a combination of chemotherapy and radiation therapy prior to surgery (neoadjuvant therapy). Patients who have had upfront surgery may be candidates to get more therapy including chemotherapy and radiation therapy. Clinical trials over the past decade show some conflicting results regarding the role of radiation after therapy (postoperative or adjuvant therapy).
One large Phase III trial called RTOG 0848 is looking at the role of adjuvant radiation therapy for resectable patients. Approximately 20 percent of patients are deemed eligible for surgery at the time of diagnosis. However, sometimes surgery ends up not being a viable option for patients in this group. For borderline resectable patients, there are benefits of chemoradiation prior to surgery. With this approach, we can treat micrometastatic and subclinical disease that doesn’t show up on scans. One approach for these patients is to use standard doses of radiation along with chemotherapy, although there is some discussion as to whether doses should be delivered by intensity modulated radiation therapy (IMRT) or stereotactic body radiation therapy (SBRT).
We’re hearing a lot more about stereotactic body radiation therapy (sbrt) recently. Why is that?
With traditional treatment, patients with early-stage pancreatic tumors generally undergo surgery followed by chemotherapy, sometimes with radiation therapy. This conventional or standard radiation is delivered in relatively low doses, usually daily over the course of five to six weeks concurrently with chemotherapy. However, with SBRT, higher doses of carefully targeted radiation are used to treat tumors, delivered in a few “fractionated” doses over the course of only five days with limited toxicity. It’s these higher doses that can potentially lead to improved local control of the disease and possibly survival. One of the big hurdles that we’ve overcome is making substantial improvements that include control of a patient’s breathing motion, since pancreatic tumors, like liver tumors, move during breathing.
One well-known type of SBRT is called the CyberKnifesystem, which is the only fully robotic radiation delivery system. The CyberKnife System’s design, along with real-time imaging, ensures that the CyberKnife System can deliver a maximum dose of radiation directly to the tumor from varying angles with sub-millimetre precision in a non-invasive way. This is done by tracking and adjusting for tumor or patient movement during the treatment to reduce how much the healthy tissue is exposed to radiation.
Other improvements include CT scan on rails, which increases the accuracy of radiation treatment by precisely identifying the location of the tumor, and MRI during radiation treatment. These technologies allow for what is called adaptive planning, which means that the radiation oncologist can adjust the focus of the beam or re-plan the treatment to account for any shift in the tumor or normal tissues.
Can you cite some studies involving sbrt?
There are currently many studies of SBRT that are showing significant promise. In a multi-institution study published in the journal Cancer, we treated 49 locally advanced pancreatic cancer patients with SBRT. The patients received SBRT in five fractionated doses and chemotherapy with gemcitabine before and after the radiotherapy treatment. Four weeks after SBRT, all 22 patients who completed a quality of life questionnaire reported an eight-point reduction in pain from a baseline measure of 25, and their quality of life stayed the same.
In a second study, published in the Annals of Surgical Oncologywe analyzed information on patients with pancreatic cancer who received SBRT and chemotherapeutic drugs between 2010 and 2014. The study looked at acute and late toxicity of SBRT in locally advanced and borderline resectable pancreatic cancer patients. Of 88 patients, eight experienced severe gastrointestinal side effects. Most of the patients who had side effects also had tumors that were invading the intestines or stomach. For this reason, tumors invading the bowel or duodenum should only get SBRT if the plan is for the patient to undergo surgery following completion of SBRT. In this study, 19 patients with tumors previously considered inoper- able (unresectable or locally advanced) were able to have surgery after SBRT.
Several patients had a complete response at the time of surgery, meaning there was no tumor left behind. This was extremely encouraging because we want to give patients a surgical option, which is especially important since pan- creatic tumors can often attach to and grow around blood vessels, making them difficult to remove. With SBRT, the tumor changes to a form that can be more easily pried away from blood vessels. Plus, the current published data show that with SBRT over five days we can provide at least equivalent or better outcomes in terms of sur- vival, with less toxicity, and better pathologic response rates than what is generally seen with standard treatment over five to six weeks. That could be an extremely important factor for patients.
Another factor to consider is that shorter treatment times with SBRT may provide other benefits such as allowing patients access to full-dose chemotherapy much more quickly than those undergoing standard radiation which, because of its longer sched- ule, potentially gives cancer more time to spread. Quality of life is also a significant factor. Most patients we treat with SBRT have tumors that can’t be removed by surgery, and that means aver- age survival is only about one year. These patients generally must go through approximately six weeks of standard chemotherapy and radiation. That can be difficult for patients and their families. However, SBRT is only about five days, so patients can spend less time getting treatment and more time enjoying their families. In addition, ongoing studies are testing combinations of SBRT with immunotherapy, and researchers are also continuing to study the effectiveness of various radiation doses used in SBRT.
What about patients who have very advanced or metastatic disease?
More than half of all pancreatic cancer patients are diagnosed with Stage IV metastatic disease that has spread to distant sites like the liver or lungs. In these instances, radiation’s role is limited, depend- ing on the patient. We have made some promising strides in managing advanced pancreatic cancer with chemotherapeutic regimens like Abraxane, or nab-paclitaxel, in combination with gemcitabine. Gemcitabine combined with Abraxane was shown to be superior to gemcitabine alone in terms of overall survival. The toxicities are known and are well-managed. FoLFIrINoX also has been shown to provide a survival advantage over gemcitabine alone. Addition- ally, some patients may benefit from radiation therapy or a nerve block to help relieve symptoms.
Do patients ever experience side effects from radiation therapy?
Yes, patients may experience side effects from radiation therapy. Each patient’s reaction to radiation therapy is unique, and it is difficult to predict which side effects a patient may experience or how severe they could be. In certain instances, some patients may experience some abdominal discomfort, appetite loss, gastrointestinal issues like diarrhea, skin changes, or fatigue because of radiation therapy. Radiation therapy can also lower blood counts, which can raise the risk of infection. However, these symptoms go away usually within a few weeks after treatment ends. Patients should contact their physician if side effects are severe or unmanageable.
Radiation may also be helpful in alleviating pain caused by the tumor and in treating patients who are elderly or aren’t healthy enough for other therapies, including surgery.
Are there any trials that particularly excite you?
I’m especially excited about the ongoing Alliance trial, which examines how well combination chemotherapy with or without SBRT before surgery works in patients with borderline resectable pancreatic cancer. SBRT may destroy more tumor cells and cause fewer side effects. In my opinion, the findings will pave the way to an improved understanding of which patients are more likely to benefit from SBRT.
Radiation implants go in and out of favor a lot. is there a role for radiation implants in pancreatic cancer treatment?
I’m involved in a small pilot study looking at the safety of an active radiological medical device that is implanted into pancreatic tumors in patients receiving standard chemotherapy treatment (gemcitabine or gemcitabine plus Abraxane). The device is called OncoSil™, which is an experimental therapy that carries the active treatment “radioactive Phosphorous (32P)” inside inactive silicon particles. Once implanted, the particles will stay in the tumor permanently with the hopes of destroying cancer cells. This is unique in that it delivers a high dose of radiation in one day and does not delay chemotherapy.
Are you hopeful about the future of pancreatic cancer treatment?
Yes, I’m very excited about new systemic therapies and new immunotherapies that are emerging and how radiation therapy can be integrated into these therapies. Patients deserve the most targeted and advanced treatments in their fight against this disease. I believe the biggest breakthrough we’ve had with radiation therapy is turning some pancreatic cancer patients whose tumors were not considered to be surgically treatable into surgical candidates. Getting more patients to surgery would be a huge step forward and could increase the number of pancreatic cancer survivors.
Make a difference to pancreatic research by donating to Lustgardentoday.

